Chemical Synthesis Equipment

Although our research focuses on non-covalent intermolecular interactions, we still routinely prepare molecules of interest for our studies through classical (covalent) synthesis methods, so of course we have a full complement of the common equipment for chemical synthesis (glassware, rotary evaporators, Schlenk lines, etc.). In particular, we have prepared smaller, more manageable model systems to compare to our very large dendrimers, and fluorescent dyes with desirable properties.
Our Department provides shared facilities such as nuclear magnetic resonance (NMR) and mass spectrometry (LC-ESI-MS, high-resolution EI-MS, MALDI-TOF).
Our Department provides shared facilities such as nuclear magnetic resonance (NMR) and mass spectrometry (LC-ESI-MS, high-resolution EI-MS, MALDI-TOF).
Diode-Array UV-Vis Spectrophotomer
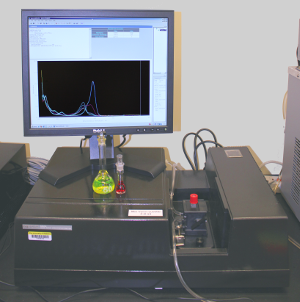
This spectrophotomer is one of the two workhorses in our group, together with the benchtop spectrofluorimeter.
The optical bench comes from the venerable and virtually indestructible Hewlett-Packard 8452a. A single-beam instrument with a 512-element diode-array detector and a high-pressure deuterium light source, it allows us to record a full spectrum in as little as 0.33 s over the range 190-800 nm with a resolution of 2 nm. The instrument is equipped with a thermostatted, stirring cell holder connected to a circulating water bath for constant-temperature work.
The software and electronics have been upgraded to modern standards by Olis, Inc., so that the instrument can be connected to any computer through a standard USB connector, and is operated with much more capable and versatile software.
The optical bench comes from the venerable and virtually indestructible Hewlett-Packard 8452a. A single-beam instrument with a 512-element diode-array detector and a high-pressure deuterium light source, it allows us to record a full spectrum in as little as 0.33 s over the range 190-800 nm with a resolution of 2 nm. The instrument is equipped with a thermostatted, stirring cell holder connected to a circulating water bath for constant-temperature work.
The software and electronics have been upgraded to modern standards by Olis, Inc., so that the instrument can be connected to any computer through a standard USB connector, and is operated with much more capable and versatile software.
Steady-State Spectrofluorimeter

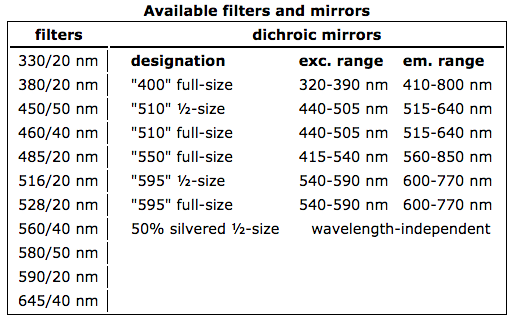
Our spectrofluorimeter is a PC1 model from ISS. The instrument has a "T" optical configuration, in that it has two independent emission channels. The first channel, and the one we used most commonly, uses a monochromator for wavelength selection. The second channel uses optical filters instead. Resolution is controlled by calibrated manual slits for maximum repeatability. The excitation channel and both emission channels are equipped with computer-controlled high-aperture Glan-Thompson calcite polarizers, so the machine is also capable of measuring steady-state fluorescence anisotropy in a single measurement. Excitation is provided by a 300 W high-pressure xenon arc lamp. Excitation correction is carried out through a rhodamine B quantum counter with a dedicated detector. Extra filters can be mounted in all channels. We also have a set of handy reflective neutral density filters that allow us to study very bright fluorophores in optimal conditions. Detection is by red-sensitive photomultiplier tubes driven in digital mode. The sample compartment is thermostatted by an external circualting water bath to carry out constant-temperature work, and it provides stirring. The sample compartment can also be flooded with inert gas if necessary.
Time-Resolved Fluorimeter (fluorescence lifetime)

We can determine fluorescence lifetimes and carry out other time-resolved fluorescence measurements using our Edinburgh Photonics MiniTau, which can determine lifetimes as short as 300 ps. Excitation is provided by an interchangeable diode laser: our current laser provides 150 ps pulses of light at 485 nm, at pulse repetition rates up to 20 MHz. Since the laser excitation light is polarized, the machine can also run fluorescence anisotropy decay measurements with the simple addition of an emission polarizer in the future. Control of the excitation power is carried out through an optical density filter wheel. Optical filters are used for emission wavelength selection. Detection uses a high sensitivity photomultiplier tube driven in digital mode.
The MiniTau uses the time–correlated single photon counting technique (TC–SPC) to acquire lifetime data. The instrument's software is capable of analysis of decay curves including up to 4 exponential decays, but we have also developed in-house tools to fit more complex decays.
Finally, the sample holder provides temperature control through an external circulating water bath for constant temperature work. The instrument is currently equipped with a solution sample holder (cuvette), but other holders are available from the manufacturer e.g. for solids.
The MiniTau uses the time–correlated single photon counting technique (TC–SPC) to acquire lifetime data. The instrument's software is capable of analysis of decay curves including up to 4 exponential decays, but we have also developed in-house tools to fit more complex decays.
Finally, the sample holder provides temperature control through an external circulating water bath for constant temperature work. The instrument is currently equipped with a solution sample holder (cuvette), but other holders are available from the manufacturer e.g. for solids.
Microwell Plate Reader

Our BioTek Synergy 2 multi-mode plate reader can measure absorbance spectra, fluorescence emission and fluorescence anisotropy, and luminescence emission. The plate reader uses a monochromator to perform spectral scanning in absorption, but uses filters for all fluorescence and luminescence measurements. It can also carry out kinetics measurements for all these detection modes. Temperature control is available from ambient temperature to 65°C. All measurements can be carried out on plates from 6–well to 1536–well microplates; we most commonly use 96–well black polystyrene plates with a clear plastic bottom to carry out both fluorescence and absorbance measurements. The instrument has proved to be a valuable time-saver, and quite a few other groups have taken advantage of this instrument. We welcome anybody that would like to try their hand at plate reading. For routine work, we have a sign-up system set up to share time on the instrument.
Isothermal Titration Calorimeter

We have recently purchased a TA NanoITC isothermal titration calorimeter equipped with a low-volume (190 μL) cell. An isothermal titration calorimeter (ITC) provides a direct measurement of the thermodynamic parameters (K, ΔH°, ΔS°) of a molecular interaction through a single experiment performed at a constant temperature, through measurement of the heat exchange caused by repeated additions of a guest to the host system under study. No labels or other analytical handles are necessary in this technique: the absorption or release of heat are a nearly universal property of any chemical transformation, so this method has an almost unlimited scope. This will allow us to perform more direct studies of interesting interactions, without having to worry about complications introduced by ancillary detection moieties.
Liposome Electroformation Apparatus

We recently started using the electroformation method to prepare Giant Unilamellar Vesicles (GUV) with relatively good yield and with minimal formation of multilamellar liposomes. Our instrumental setup was developed in-house. It consists of a chamber made from two ITO glass slides sandwiching a thin non-conducting spacer. The core of the setup is a HP 3311A function generator, which provides the oscillating electric field (a few Vpp RMS) that is applied across the chamber through the ITO electrodes.
We monitor a number of parameters during each liposome synthesis run. A Tektronix 2246 oscilloscope is used to monitor the voltage and frequency of the field applied to the chamber; a BK Precision 2709B handheld multimeter capable of measuring microamperes is connected in series with the chamber to monitor that only a very small current is flowing in the system. The exact temperature of the chamber is not critical, as long as it is kept above the Tm of the lipids in use. We simply lay our chamber on a lab hot plate and we monitor the temperature from time to time during the run with a handheld platinum resistance thermometer.
We monitor a number of parameters during each liposome synthesis run. A Tektronix 2246 oscilloscope is used to monitor the voltage and frequency of the field applied to the chamber; a BK Precision 2709B handheld multimeter capable of measuring microamperes is connected in series with the chamber to monitor that only a very small current is flowing in the system. The exact temperature of the chamber is not critical, as long as it is kept above the Tm of the lipids in use. We simply lay our chamber on a lab hot plate and we monitor the temperature from time to time during the run with a handheld platinum resistance thermometer.
Liposome Extruder

We use the standard extrusion method to prepare highly uniform samples of Large Unilamellar Vesicles (LUVs, with a diameter between 50 nm and 200 nm), by repeated extrusion of a lipid suspension in water through a polycarbonate membrane with calibrated pores. The raw lipid suspension to be extruded is obtained by rehydration of a lipid film. Our commercial small-scale manual extruder was made by Avanti Polar Lipids.